[
]
)
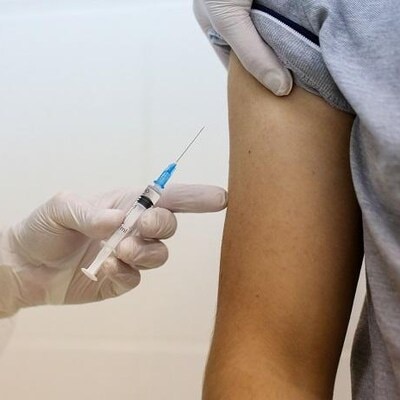
Representative Image: The injectable version also had a safety and efficacy profile similar to the IV formulation in patients with RMS and PPMS. Photo: Bloomberg
The US Food and Drug Administration on Thursday approved Roche’s under-the-skin injection to treat patients with multiple sclerosis.
The therapy is already approved as an infusion or IV therapy for multiple sclerosis, which is given twice a year, under the brand name Ocrevus.
The approval for the subcutaneous, or under-the-skin, version of the therapy will help expand its use to treatment centers not equipped to handle intravenous therapies.
Ocrevus is used to treat patients with relapsing multiple sclerosis (RMS) and primary progressive multiple sclerosis (PPMS).
Roche did not immediately respond to a Reuters’ request seeking details on availability date and pricing of the injectable version.
Multiple sclerosis is a condition that occurs when the immune system attacks the brain and spinal cord.
Roche estimates that this disease affects more than 2.8 million people worldwide.
RMS is characterized by episodes of new or worsening signs or symptoms, followed by periods of recovery. PPMS causes gradual worsening of neurological symptoms and disability without relapses or remissions.
The subcutaneous version, Tecentriq Hybreza, is a 7-minute injection given twice a year, the same schedule as the previously approved intravenous infusion.
The approval was based on data from a late-stage study that showed Ocrevus as an under-the-skin injection was non-inferior to its intravenous version, as measured by blood levels over 12 weeks.
The injectable version also had a safety and efficacy profile similar to the IV formulation in patients with RMS and PPMS.
The subcutaneous formulation, which received marketing authorization from the European Commission in June, combines Ocrevus with Halozyme Therapeutics’ drug delivery technology, which allows the therapy to be rapidly dispersed and absorbed into the bloodstream.
Ocrevus is a monoclonal antibody designed to target CD20-positive B cells, a specific type of immune cell thought to be a key contributor to nerve cell damage.
More than 350,000 people with multiple sclerosis have been treated with Ocrevus IV globally, according to Roche. The IV formulation of Ocrevus recorded sales of 6.38 billion Swiss francs ($7.54 billion) in 2023.
(Only the headline and picture of this report may have been reworked by the Business Standard staff; the rest of the content is auto-generated from a syndicated feed.)
First Published: Sep 13 2024 | 9:06 AM IST